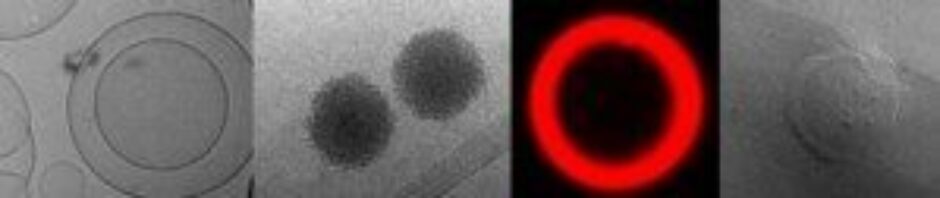
Elias Fattal is a full professor in Drug Delivery Science at the University of Paris-Saclay in Châtenay-Malabry, France, and has been President of APGI from 2003 to 2010. He received his Pharmacy Degree (1983) and Ph.D. (1990) from the University of Paris-Sud and followed an internship in Hospital Pharmacy at the University of Lille (1984-1986). After visiting the Department of Pharmaceutical Chemistry at the University of California, San Francisco for a post-doctoral position (1990-1991), he became Associate Professor (1992) and full Professor at the University of Paris-Saclay (2000). He has been the head of the Institut Galien Paris-Saclay (2010-2019). Over the past 25 years, he has made fundamental and applied contributions to the fields of drug delivery using nanotechnologies for targeted or local delivery of drugs and nucleic acids. He has been recently focusing on lung nanotoxicity as well as on the design of nanoparticle-based delivery systems for the delivery of anti-inflammatory drugs and nucleic acids. Several of his patents have been licensed to the pharmaceutical industry. One of his patents led to Calixarene® Cevidra, a cream for the treatment of external actinide contamination. He has contributed to around 300 publications and book chapters. Prof. Fattal has received the Pharmaceutical Sciences World Congress (PSWC) Research Achievement (2007), the Controlled Release Society fellow award (2016), was awarded in 2016 by the French Academy of Sciences for his research at the interface of chemistry and biology and more recently in 2018 received the Maurice-Marie Janot Award. He serves on the editorial board of several scientific journals and is a member of the National Academy of Pharmacy, the National Academy of Medicine and the European Academy of Sciences.
Elias Fattal is a full professor in Drug Delivery Science at the University of Paris-Saclay in Châtenay-Malabry, France, and has been President of APGI from 2003 to 2010. He received his Pharmacy Degree (1983) and Ph.D. (1990) from the University of Paris-Sud and followed an internship in Hospital Pharmacy at the University of Lille (1984-1986). After visiting the Department of Pharmaceutical Chemistry at the University of California, San Francisco for a post-doctoral position (1990-1991), he became Associate Professor (1992) and full Professor at the University of Paris-Saclay (2000). He has been the head of the Institut Galien Paris-Saclay (2010-2019). Over the past 25 years, he has made fundamental and applied contributions to the fields of drug delivery using nanotechnologies for targeted or local delivery of drugs and nucleic acids. He has been recently focusing on lung nanotoxicity as well as on the design of nanoparticle-based delivery systems for the delivery of anti-inflammatory drugs and nucleic acids. Several of his patents have been licensed to the pharmaceutical industry. One of his patents led to Calixarene® Cevidra, a cream for the treatment of external actinide contamination. He has contributed to around 300 publications and book chapters. Prof. Fattal has received the Pharmaceutical Sciences World Congress (PSWC) Research Achievement (2007), the Controlled Release Society fellow award (2016), was awarded in 2016 by the French Academy of Sciences for his research at the interface of chemistry and biology and more recently in 2018 received the Maurice-Marie Janot Award. He serves on the editorial board of several scientific journals and is a member of the National Academy of Pharmacy, the National Academy of Medicine and the European Academy of Sciences.